How Do Electronegativity And Polarity Relate
Periodic trends in electronegativity Electronegativity polar covalent bonds compounds explain differences 8.4: bond polarity and electronegativity
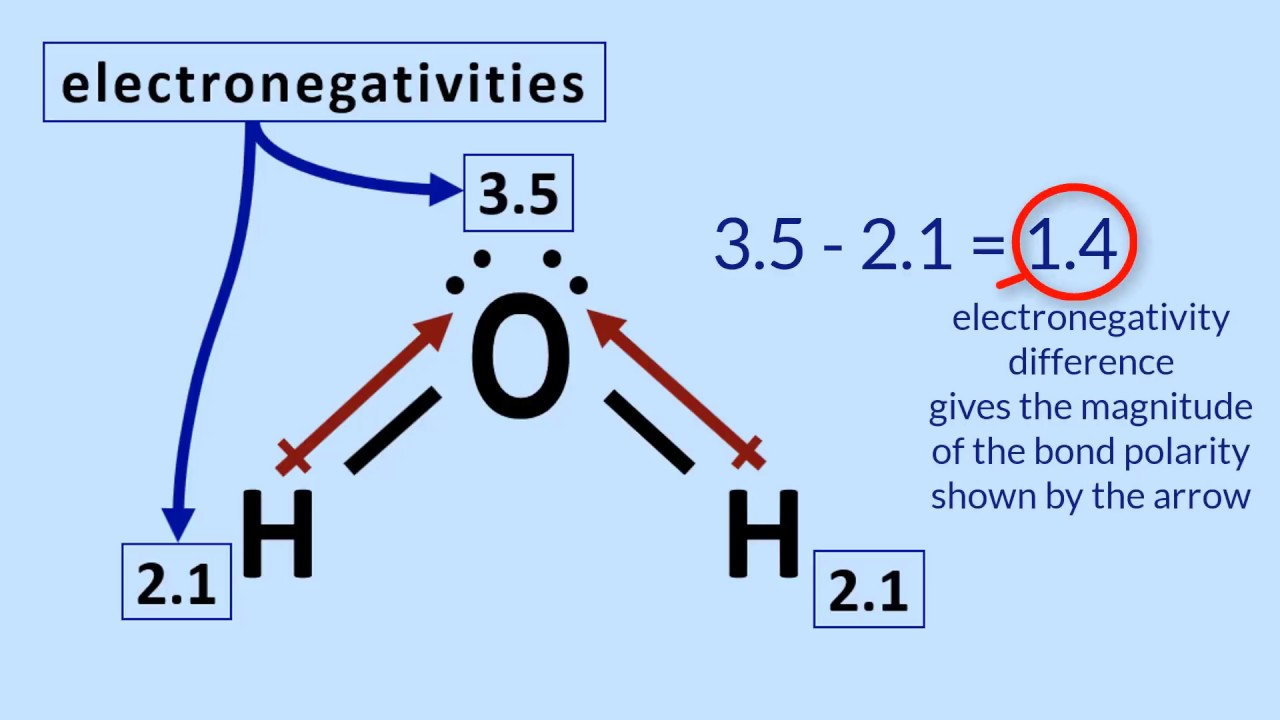
4.2 Relative polarity of bonds from electronegativity values [SL IB
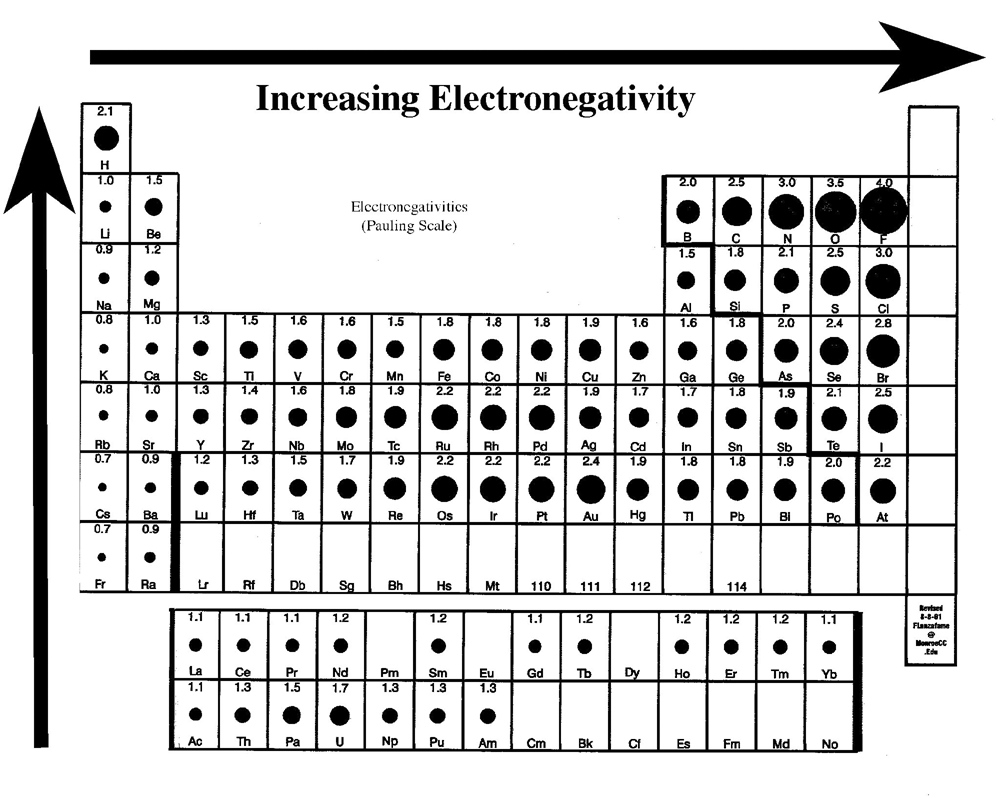
Periodic electronegativity trend electron affinity period table go across chemistry trends presentation electronegativities sliderbase do increase orbitals when diagram electrons Chemical compound Electronegativity difference bond type
Nitrogen electronegativity
Electronegativity differences explain polar bonds in covalent compoundsPeriodic table 4.2 relative polarity of bonds from electronegativity values [sl ibElectronegativity difference bond type.
What trend in electronegativity do you see as you go across a periodCovalent polar bond ionic bonding bonds polarity properties electron molecules electronegativity hydrogen atoms nonpolar polaire potential libretexts covalente structures molecular Electronegativity periodic greatest covalent radius atomic ionic atom electronegative chloride lowest presentation appears electrons increases atomsElectronegativity periodic table bond chemistry polarity pauling elements energy values 3d general principles chart bonding scale ionization applications patterns trends.

Chemical bonding: periodic trends
Chemistry bond electronegativity ionic binding verschil covalente covalent teaching polar bonding table polaire gradation een periodic kids choose boardPeriodic table electronegativity trend Stoichiometric basics: chemistry for kids!6.2 electronegativity and polarity – chemistry fundamentals.
Electronegativity periodic trends ionization energy chart nc license ccElectronegativity polarity relative ib chemistry values bonds Electronegativity polarity chartElectronegativity and polar covalent bonding.

Electronegativity range
Electronegativity pauling periodic table trends values chemistry bonds scale linus electronic number printable atomic period noble gases chemical chart valueElectronegativity oxidation table number chemistry introduction highest lowest elements bottom left right top Electronegativity and bond polarityElectronegativity polarity chart.
Electronegativity and bond polarityElectronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common Electronegativity polarity chartExplain the relationship between electronegativity difference and polarity.

Which diagram best represents a polar molecule
How can i determine bond polarity? + examplePolarity electronegativity bond chemistry Polar which diagram polarity bond molecule represents dipole electronegativity moment chemistry problems practiceElectronegativity chart and ionic character.
Electronegativity polarity chartBond polarity chart Electronegativity and polarityElectronegativity periodic chemical trends compound electron atoms pairs bonds electronegativities ions.
Electronegativity and oxidation number
Covalent bonding – introductory chemistryElectronegativity periodic table trend chemistry element chart energy ionization labeled google saved printable organic Chapter 5.6: properties of polar covalent bondsElectronegativity periodic trends bonding chemical trend chart element polarity bond electrons tendency atom electronegative table increasing electron attraction chemistry attract.
Electronegativity and polarity study guide .
4.2 Relative polarity of bonds from electronegativity values [SL IB

6.2 Electronegativity and Polarity – Chemistry Fundamentals

Electronegativity Chart And Ionic Character

3.3 Electronegativity | Atomic combinations | Siyavula

Periodic Table Electronegativity Trend | Ionization energy
How can I determine bond polarity? + Example

Electronegativity and Polar Covalent Bonding - dummies